Is your contraceptive affected by the Supreme Court's TRO?
Groups are calling on the Supreme Court to lift the temporary restraining order that has prevented the Department of Health (DOH) from procuring and distributing certain types of contraceptives.
The order has also prohibited the Food and Drug Administration (FDA) from issuing new certificates of product registration (CPR) for these products, all of which are used by women.
Commission on Population executive director Juan Antonio Perez warned that by 2020, 47 female contraceptive brands will be off the shelves. In a media forum last week, he revealed that progestin-only pills are already gone from the market.
Read: Progestin-only pills will disappear from shelves by mid-2017 –POPCOM
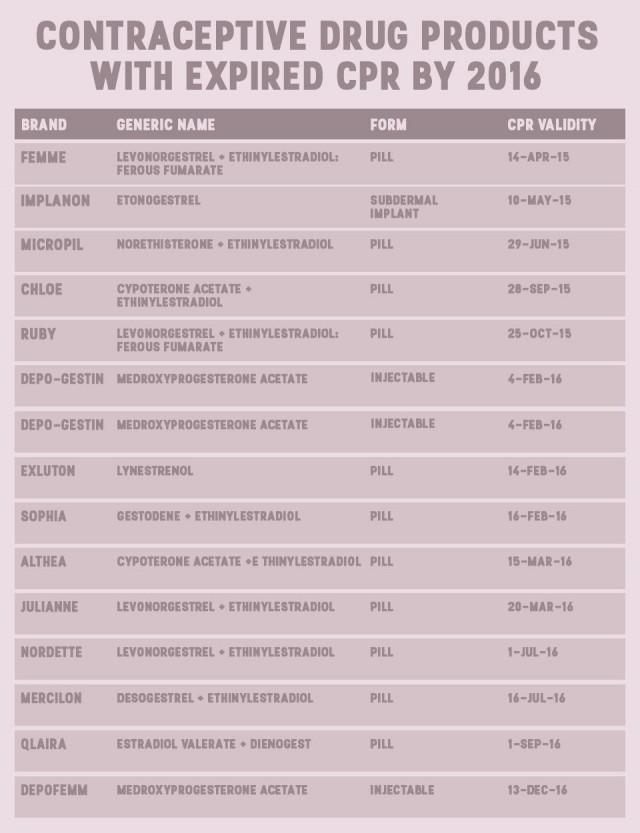
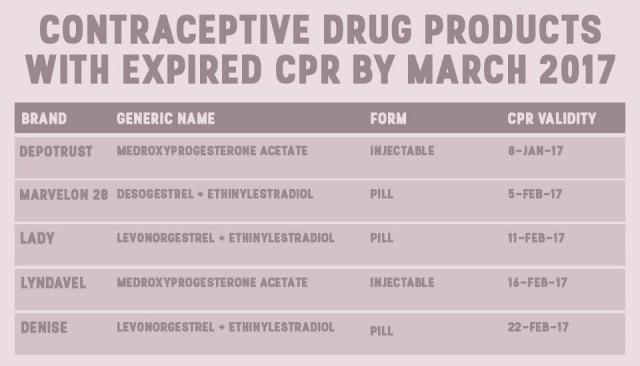
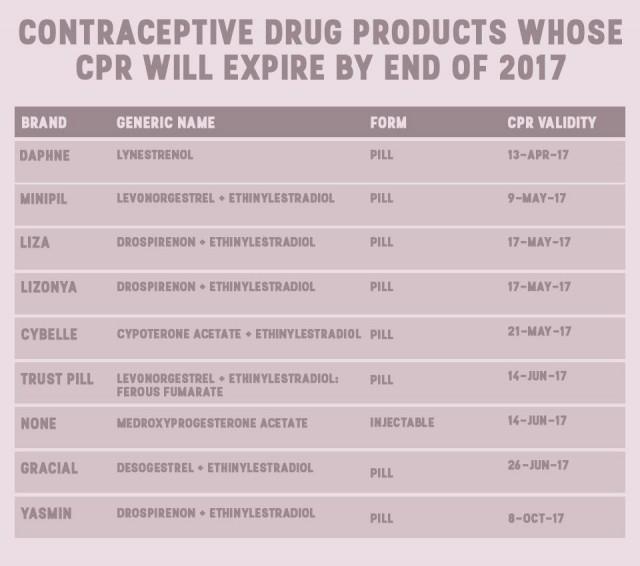
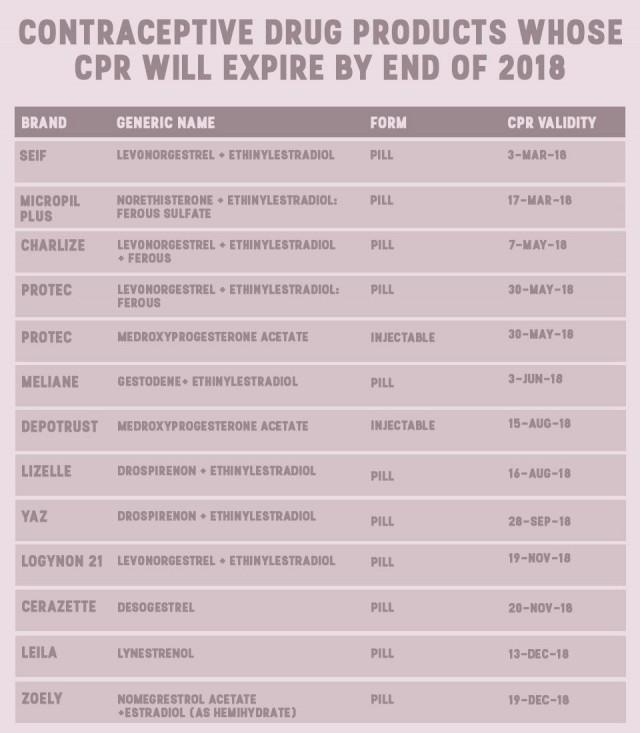
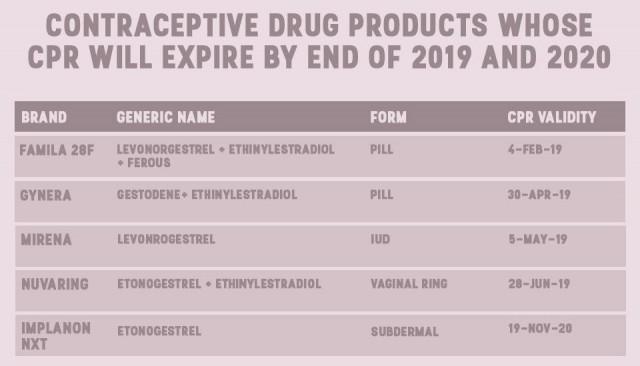
Their data, shared with GMA News Online via email, showed that by the end of 2017, eight brands of pills and one brand of injectable will also be unavailable for consumers.
Perez said products are registered by brands, which means that their CPRs will expire at different times. Their data shows that the Nuvaring, a vaginal ring, and subdermal implant Implanon NXT will be the last two to go.
Reproductive health advocates have called on the Supreme Court to "stop trampling on women's rights" and act on the petition filed by the DOH and the Office of the President last March to immediately lift the TRO.
Read: RH advocates want contraceptives TRO lifted, say legal limbo baseless
The Supreme Court issued the TRO in 2015, first only covering Implanon and similar implants. It was later expanded to cover pills, injectables, intrauterine devices, vaginal rings, and other brands
The petition was filed by Alliance for the Family Foundation, Philippines, Inc. (ALFI), in protest of the Food and Drug Administration's (FDA) the issuance of certificates of product registration to Implanon and Implanon NXT, contraceptive devices which they claim may induce abortions.
The court outlined in the TRO instructions for the FDA and the Department of Health to formulate rules and regulations for the screening, evaluation and approval of all contraceptive drugs and devices, as well as the purchase and distribution or dispensation of the products while "allowing the petitioners [ALFI] to be heard" in the process.
DOH spokesperson Enrique Tayag has expressed confidence that the Supreme Court will lift the TRO and respect the rights of women. —JST, GMA News



