Pharma company warns of counterfeit anti-rabies serum Equirab
A pharmaceutical company has warned the public against the counterfeit drugs bearing the name of its anti-rabies serum Equirab which is being circulated in the local market.
BSV BioScience Philippines said Equirab is a drug indicated for passive immunization against rabies. It is used for the “prevention of rabies in patients at risk of being exposed to rabies after contact with a rabid animal or animal presumed to be rabid.”
The Philippine Food and Drug Administration (FDA) in August 2021 already issued an advisory on the said counterfeit product which “poses potential danger or injury to consumers.”
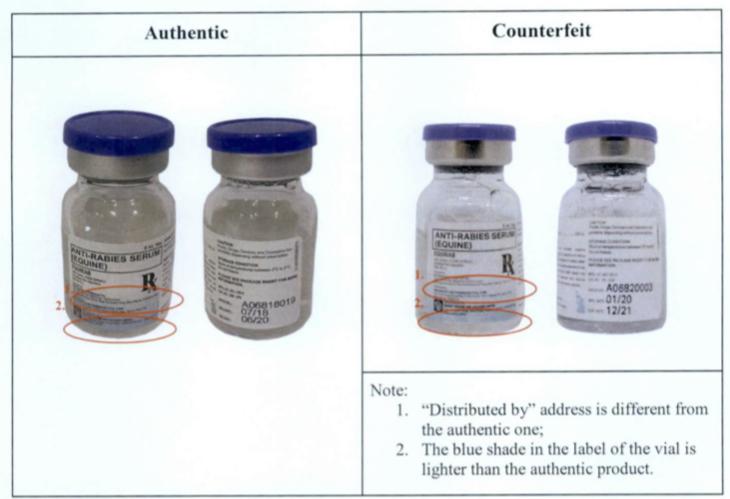
To identify the difference between the counterfeit and authentic drug, the FDA noted the following:
- “Distributed by” address is different from the authentic one
- The blue shade in the box is lighter than the authentic product
- 2D barcode is not readable and coding pattern is different
Along with the FDA, BSV BioScience said they obtained fake samples of Equirab in its 200 IU/ml (1000 IU/5 mL) formulation.
“Patients could be at risk of contracting rabies and potentially may suffer once injected with a counterfeit product when they happen to have been exposed to a rabid animal,” the company said.
BSV BioScience said it would seek penalize the manufacturers and distributors of counterfeit Equirab to the “full extent of the law.”
“BSV is currently exhausting legal options with the government's help to curb the manufacture and distribution of counterfeit Equirab. Ongoing operations have already pinpointed alleged counterfeiters and have led to ongoing legal cases against them,” it said.
According to FDA, the importation, selling, or offering for sale of such is in direct violation of Republic Act (RA) Number 9711 or the Food and Drug Administration Act of 2009, and the RA No. 8203 or the Special Law on Counterfeit Drugs.
FDA also urged the local government units and law enforcement agencies to ensure that such a product is not sold or made available in their areas of jurisdiction.
The public, healthcare professionals, and other outlets, were then reminded to purchase drug products only from FDA-licensed establishments.—Giselle Ombay/AOL, GMA News



