Janssen, Clover COVID-19 vaccines secure Ethics Review Board approval
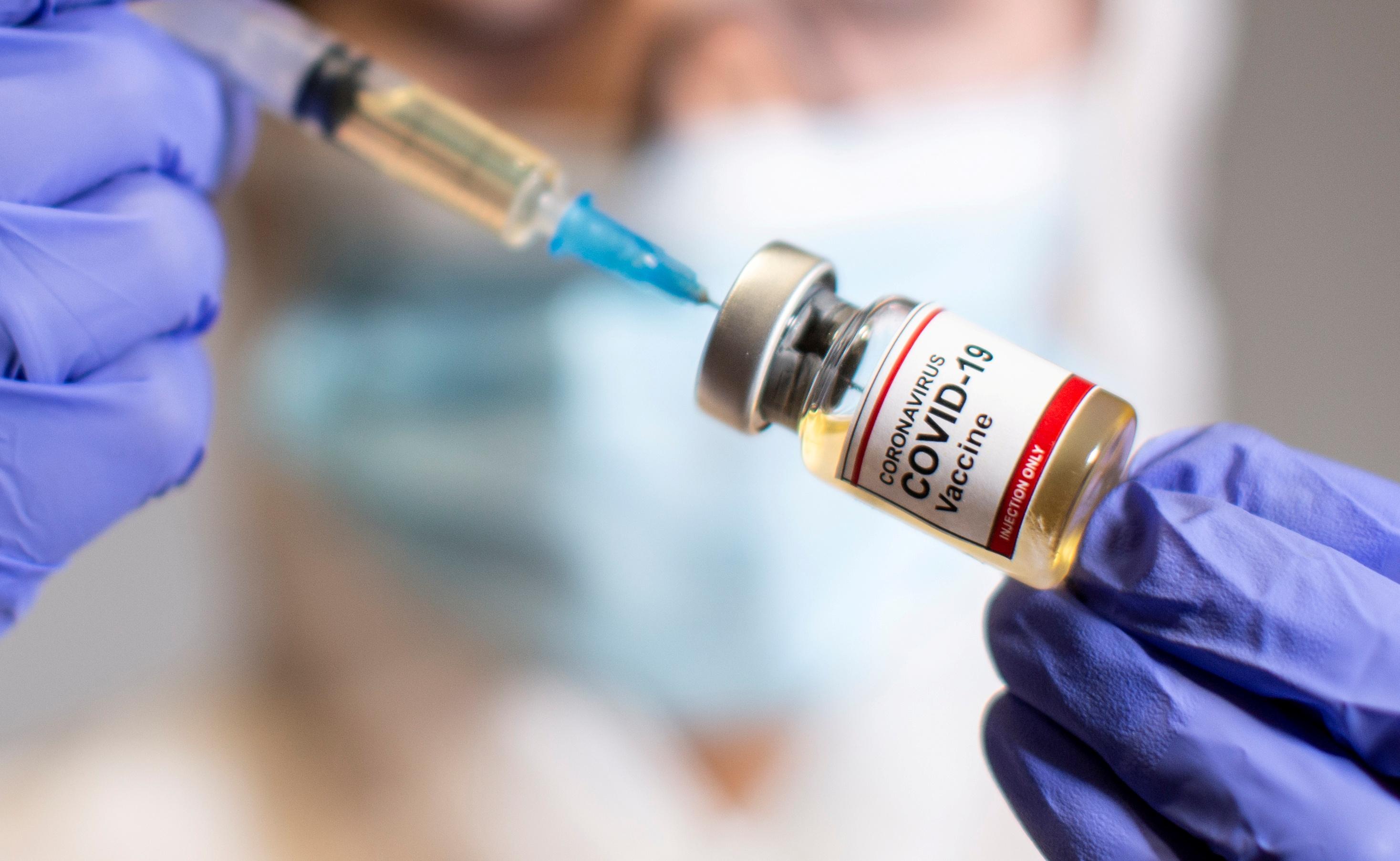
Janssen and Clover COVID-19 vaccines have secured the approval of the Ethics Review Board, Health Undersecretary Maria Rosario Vergeire said Monday.
Vergeire said that Janssen, a COVID-19 vaccine developed by Johnson and Johnson, and Chinese firm Clover joined AstraZeneca in the list of COVID-19 vaccines which already secured the nod of the Ethics Review Board.
The Health official, however, stressed that these COVID-19 vaccines would need the approval of both the Ethics Review Board and the Vaccine Experts Panel (VEP) under the Department of Science and Technology before these can be evaluated by the Food and Drug Administration (FDA).
The VEP under DOST reviews the Phase 1 and 2 clinical trials of the candidate vaccine while the Ethics Board evaluates the selection for participants for human clinical trials, among other safeguards that the vaccine manufacturer provided for the participants.
“Parallel po ang pag-aaral ng VEP at ng Ethics Review Board,” Vergeire said in an online forum.
Once FDA clearance is secured, these vaccines can now be legally administered in the Philippines.
Of the three that already got Ethics Review Board approval, only Clover has been given the green light by the VEP under DOST.
Chinese firm Sinovac has also got the VEP endorsement but it is yet to get clearance from the Ethics Review Board.
Only COVID-19 vaccines made by American firms Pfizer-BioNTech, Moderna, and British firm AstraZeneca-Oxford University have been proven at least 90% effective after human trials so far.
COVID-19 vaccines of Pfizer-BioNTech and Moderna, however, need a -70 to -80 degrees Celsius ultra low freezer for storage to ensure the vaccines’ effectivity—a challenge for countries with limited resources like the Philippines. Health Secretary Francisco Duque III said on Monday that the Philippine government does not have ultra low freezer storage at the moment.
Despite the clear challenge, Vergeire said that Philippine authorities are still working on securing a confidentiality disclosure agreement with Pfizer and Moderna in hopes of securing COVID-19 vaccine supply from these American firms.
Vergeire, however, said she could not say if these companies will conduct clinical trials in the Philippines or not since this could be covered by the CDA which is still under negotiation.
The Philippines already secured 2.6 million doses of AstraZeneca-Oxford COVID-19 vaccines via a tripartite agreement with the private sector and AstraZeneca, with the private sector shouldering the cost.
The 2.6 million doses, however, are only good for one million people. The Philippine population is around 110 million.
AstraZeneca is also bidding to conduct a clinical trial in the Philippines.
The Philippines has registered 439,834 COVID-19 cases so far. Of this number, 408,634 recovered while 8,554 died.
The number of active COVID-19 cases is at 22,646.—AOL, GMA News



